
#Hrd testing trial
The trial was sponsored by GlaxoSmithKline, the manufacturer of niraparib.

In one of the new trials, an international trial called PRIMA, the PARP inhibitor niraparib was evaluated as maintenance therapy for women with newly diagnosed ovarian cancer.

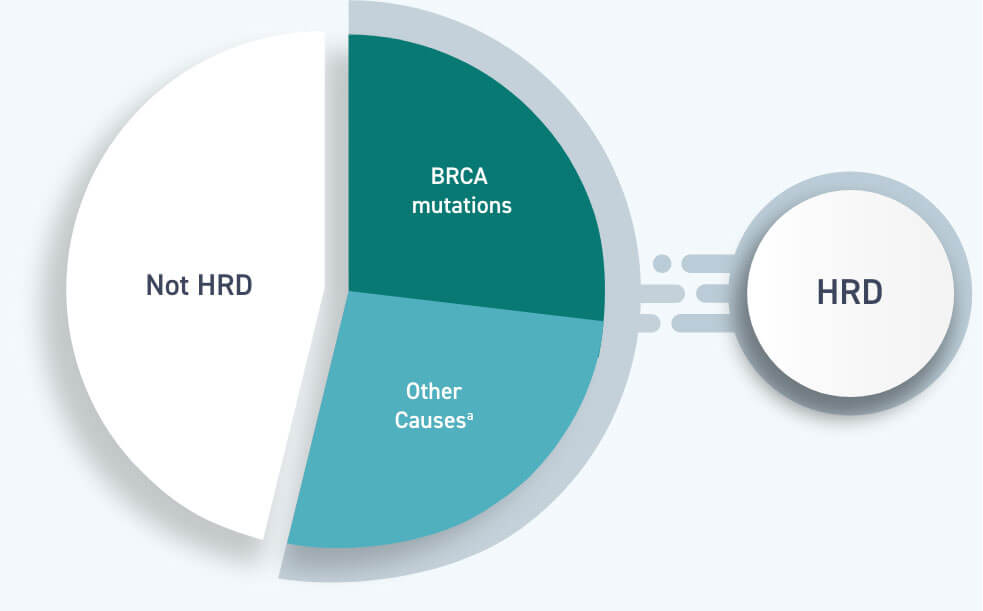
The three new clinical trials begin to address these questions. Since then, doctors questioned whether PARP inhibitors also benefit the larger group of women with HRD tumors and women with ovarian tumors that are not HRD. In 2018, a landmark clinical trial showed that maintenance therapy with the PARP inhibitor olaparib benefitted women with ovarian cancer that had a harmful BRCA mutation. HRD is defined as the presence of a harmful BRCA mutation or a certain score on a molecular test. Tumors that lack this crucial DNA repair ability are called homologous recombination deficient, or HRD. Harmful mutations in the BRCA1 and BRCA2 genes, for example, lead to faulty DNA repair. One in three women with ovarian cancer has tumors that are unable to repair a certain kind of DNA damage. “After decades studying different chemotherapy approaches, it is the first time we have meaningfully prolonged progression-free survival and hopefully we will improve long-term outcome,” Ana Oaknin, M.D., of Vall d' Hebron Institute of Oncology in Barcelona, an investigator on one of the trials, said in a news release.Ĭhristina Annunziata, M.D., Ph.D., of the Women’s Malignancies Branch in NCI's Center for Cancer Research, who was not involved in the studies, said that these results are exciting, but she noted that differences among the study designs and outcomes raises questions about how best to incorporate the different PARP inhibitors into treatment strategies for women with ovarian cancer.Įxperts are currently working together to draft new guidelines for ovarian cancer treatment, Dr. Results from all three trials were presented recently at the European Society for Medical Oncology (ESMO) Congress 2019 in Barcelona, Spain. In all three studies, the use of a PARP inhibitor as first-line therapy, maintenance therapy, or both substantially delayed the length of time before participants’ cancers came back or got worse. But for most patients, the cancer still returns within 3 years of initial treatment. Standard initial treatment for women with newly diagnosed advanced ovarian cancer typically includes first-line therapy with a combination of surgery and chemotherapy, sometimes followed by maintenance therapy-an additional treatment intended to help prevent the cancer from coming back. The studies-which tested the PARP inhibitors niraparib (Zejula), olaparib (Lynparza), and veliparib, respectively-involved women with high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer. Now, results from three new clinical trials show that the drugs might also benefit women who are newly diagnosed with advanced ovarian cancer. The approval was based on findings from the PAOLA-1 trial, results of which are described in this Cancer Currents story.ĭrugs known as PARP inhibitors are used to treat some women with advanced ovarian cancer that has returned after earlier treatment. These alterations must be determined using a specific FDA-approved test, the agency explained in a statement. In addition, the olaparib–bevacizumab treatment can be used only to treat women who have certain genetic alterations. The approval covers the use of olaparib in combination with bevacizumab (Avastin) in women whose tumors shrink partially or completely after initial treatment with chemotherapy containing a platinum drug. UPDATE: On May 8, 2020, the Food and Drug Administration (FDA) expanded the approval of olaparib (Lynparza) for the initial treatment of women with advanced ovarian cancer.


 0 kommentar(er)
0 kommentar(er)
